Please tell me the difference between indicating electrode and comparative electrode.
We will explain below.
・What are indicator electrodes?
When attempting to measure a solvent’s pH or oxidation-reduction potential, glass electrodes and metal electrodes change their electrode potential in a fixed relation to the concentration of the solvent.
・What are reference electrodes?
These are standard electrodes used to measure the electromotive force of indicator electrodes, and they maintain a fixed electrical potential unrelated to the solvent’s pH and the concentration of the oxidant and reducing agent.
They are also called comparison electrodes or collated electrodes.
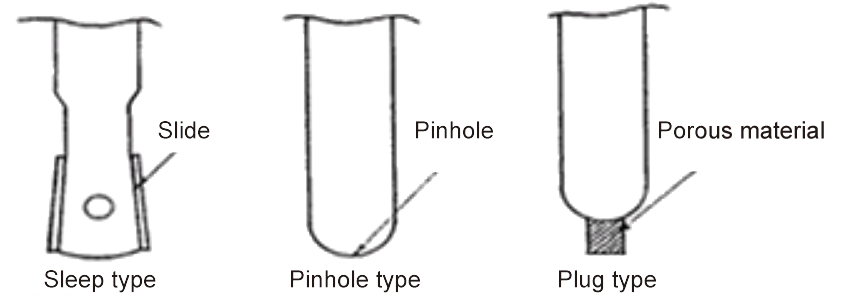
A feature of reference electrodes is their liquid junction, and when the internal liquid and test solution come into contact in this liquid junction the internal pole of the reference electrode and the test solution become electrically connected.
A representative structure of the liquid junction is shown in the figure below.
In the potentiometric titration apparatus, each of the above electrodes is immersed in a solution, and the potential difference indicated by the electrode is measured to find the end point.


