Principles of Karl Fischer Moisture Measurement
What is the Karl Fischer Method?
The Karl Fischer method, discovered by German chemist Karl Fischer in 1935, is a method for measuring moisture through titration. He discovered that iodine reacts selectively with water by using a certain reagent. The reagent was called the Karl Fischer reagent after the name of the discoverer.
There are two main methods for the Karl Fischer method.
The first is the "volumetric titration method" (or volumetric method). In this method, Karl Fischer's reagent is added dropwise, and the moisture content is determined from the volume of the reagent added dropwise to the endpoint.
The second is the "coulometric titration method" (or coulometric method). This method uses an electrolyte based on Karl Fischer's reagent. The reagent is placed in a sealed titration cell in advance, and a sample is added. Then, depending on the amount of water contained in the added sample, iodine is generated from the electrolyte by electrolysis, which is the endpoint of the reaction. The amount of electricity required for this (amount of iodine generated) is converted to the amount of water content.
Principle of the Karl Fischer Volumetric Titration Method
The Karl Fischer chemical equation and method explanation:
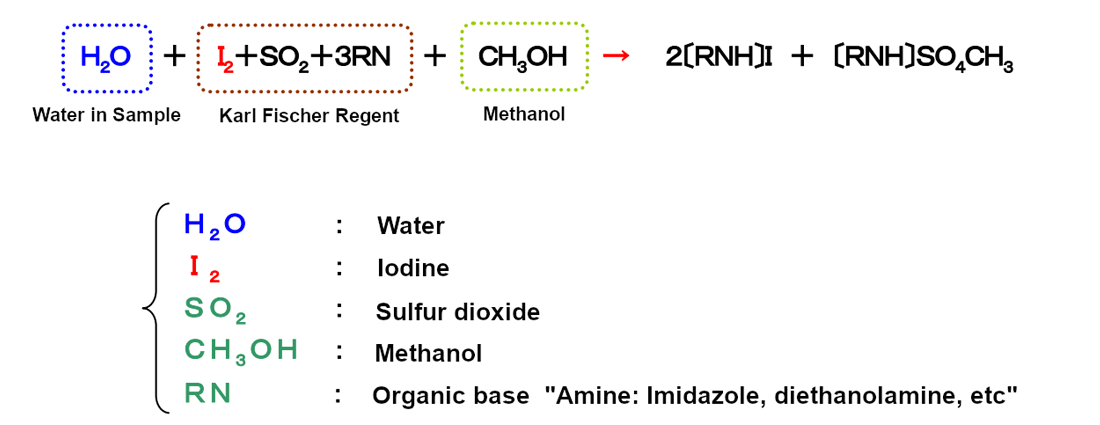
There is no water (H2O) in the post-reaction equation on the right side of the arrow. Water is consumed in the reaction. As shown in the equation, water and iodine react in a fixed ratio (1:1).
Expressing the amount of water in a sample in terms of concentration (%), the formula is as follows

The potency of the reagent can be easily understood as the amount of water (mg) that 1 mL of Karl Fischer reagent can react with. Note the units of potency (mgH2O/mL). It represents the amount of water (mg) in a volume of reagent (1mL). By multiplying this "titer" by the "titrant volume (mL)" of the amount of reagent added up to the end of the reaction (endpoint), the amount of water in mg can be obtained. The obtained moisture content (mg) is further divided by the weight of the entire sample (*) and multiplied by 100 to obtain the moisture content in %.
(*): The sample amount is multiplied by 1000 to make it a mg unit.
Principle of Karl Fischer Coulometric Titration
The reaction used to consume water in the coulometric method is the Karl Fischer reaction, the same as in the volumetric method.
The difference is that iodine (I2), a component of the Karl Fischer reagent, is not included from the beginning, but is generated by electrolysis and supplied to the Karl Fischer reaction. In the electrolytic method, "iodine ion (2I-)" is included instead of "iodine (I2)" in the Karl Fischer reagent.
2I-+ SO2+3RN : Electrolyte (generator solution)
When this "iodine ion (2I-)" is oxidized by electrolysis, electrons (2e-) are extracted as follows:
2I- → I2 + 2e-
This iodide ion becomes "iodine". The iodine produced is immediately fed to the Karl Fischer reaction and reacts with water.
This makes it the same as the Karl Fischer reagent in the reaction equation for the volumetric method and the same reaction in the coulometric method.
*Some electrolytes (generating solutions) used in the volumetric method have methanol added beforehand.
Iodine produced by electrolysis is proportional to the amount of electricity, in accordance with Faraday's Law.
The reaction of water and iodine is 1:1, requiring 2 moles of electrons to produce 1 mole of iodine.
The amount of electricity equivalent to one mole of electrons is 96485 coulombs according to Faraday's constant, and one mole of water is 18.02 g. Therefore, 1 mg of water is equivalent to (96485 x 2)/(18.02 x 1000) = 10.71 coulombs.
This results in the following formula for the amount of moisture in a sample expressed as a percentage (%).
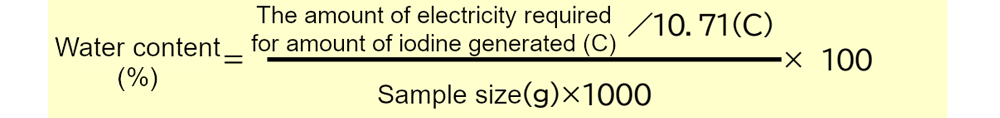
Note: 10.71 coulombs is the amount of electricity produced by a current of 107.1 mA for 100 seconds.
The amount of electricity added up to the point where the reaction ends (endpoint) can be determined and applied to the above equation to obtain the amount of water. In the numerator of the fractional part of the equation, the amount of water can be obtained in mg by dividing the amount of electricity required by the amount of electricity equivalent to 1 mg of water. Furthermore, by dividing by the weight of the entire sample (*) and multiplying by 100, the percentage of water can be obtained in units of %.
(*): The sample amount is multiplied by 1000 to make it a mg unit.
The principle of measurement and other information are based on the general concept, and may differ from your specific case. Please note that Karl Fischer reagents and other reagents may have different compositions depending on the sample, equipment, etc.


